Generation 3
Home / Glove testing system and measuring instruments / Glove testing systems / Generation 3
MK Glove Test Device Generation 3
MK can help you meet the requirements of Annex 1 glove leak testing with minimal impact on your production schedules with the MK Generation 3 glove tester:
- Integrity testing of the barrier systems, and leak testing of the glove system (…) performed minimum at the beginning and end of each batch is possible in an easy and fast way
- GITS®4 Software compliant with GAMP 5, CFR 21 Part 11 and EU Annex 11
- FDA and cGMP compliant
- ISO:9001 certified
- Customizable to your port size
- 100% Made in Germany
- Easy cleaning, H2O2/VHP fumigable
- Complete qualification service
- Usable as stand-alone or fully integrated system
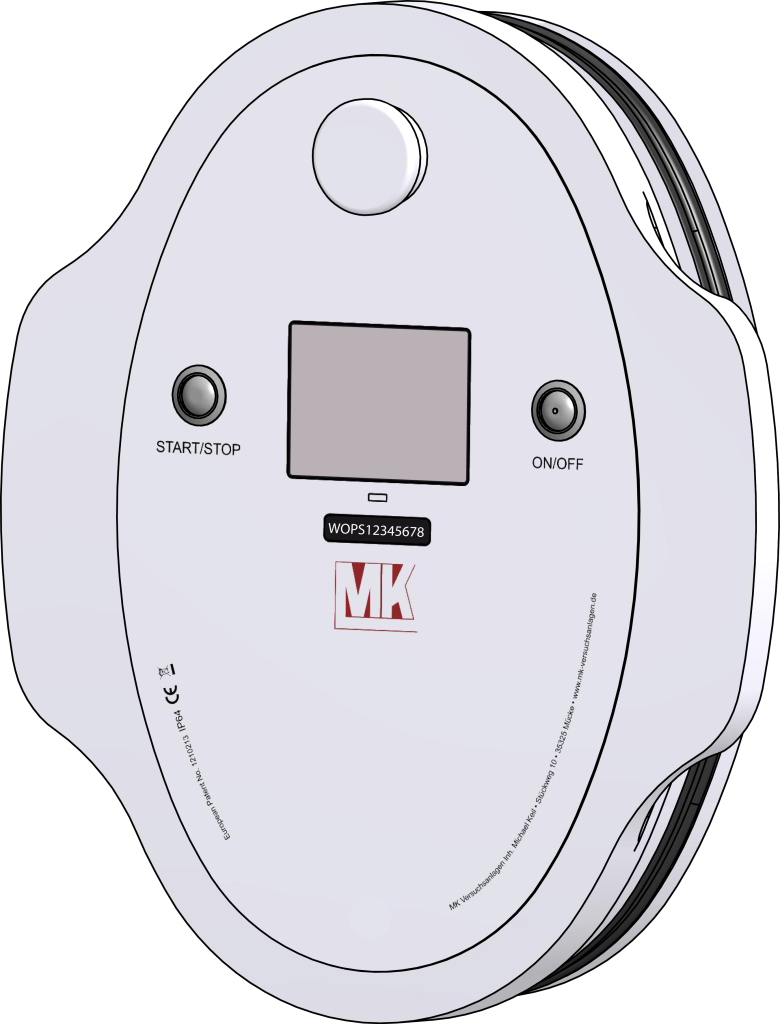
Generation 3
Total test time
- 8 – 15 Minutes (depending on glove material and port size)
Software features
MK Glove Integrity Test System – GITS®
- Traceability of glove testing
- Port and glove recognition with integrated RFID technology
- Automatic machine validation and generated reports
MK Glove Life Cycle System – GLCS®
- Monitoring of glove aging process
- Recognize glove failure in advance
- Glove life cycle tracking, from inventory to sterilization, through testing to replacement